The search for a more efficient and powerful electric car battery may have taken a significant step forward. Scientists from a Japanese university presented their work on lithium-air batteries at a meeting of the American Chemical Society this week. The researchers say they have made new progress on a so-called breathing battery that has the potential to one day replace the lithium-ion technology in electric vehicles.
“Lithium-air batteries are lightweight and deliver a large amount of electric energy,” said Nobuyuki Imanishi, a Ph.D student at Mie University. “Many people expect them to one day be used in electric vehicles.”
The lithium-air battery, versions of which have been around since the 1970s, uses air to oxidize the lithium rather than a conventional cathode. This makes the rechargeable battery lighter, and also potentially more powerful than the conventional lithium-ion battery. Lithium-air batteries have very high energy density, said to be comparable to that of gasoline.
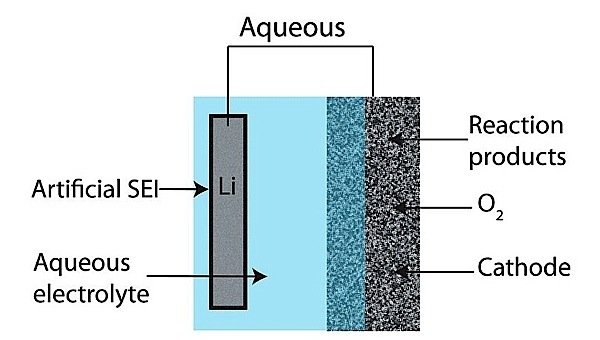
One type of electrolyte used in lithium-air batteries is water. The aqueous lithium-air battery has a lithium anode, a carbon cathode, and an aqueous electrolyte, consisting of water and dissolved lithium salts. The advantage of the aqueous design is that the lithium is protected from gases in the atmosphere and can react quickly at the air electrode.
A drawback of the aqueous type lithium-air battery is that lithium reacts with water, leading to the buildup of a solid electrolyte interphase (SEI), which leads to capacity fade over thousands of charge-recharge cycles. The lithium must therefore be insulated from the water by means of a solid barrier, usually glass or ceramic. These, however, reduce the power of the battery.
To deal with the issue of lithium’s vulnerability to SEI buildup, the Japanese team developed a layered approach to protecting the lithium, sandwiching a polymer electrolyte with high conductivity and a solid electrolyte in between the lithium electrode and the watery solution. The result, according to their press statement, was a unit “with the potential to pack almost twice the energy storage capacity, as measured in Watt hours per kilogram (Wh/kg), as a lithium-ion battery.”
Researcher Imanishi explained, “Our system’s practical energy density is more than 300 Wh/kg, That’s in contrast to the energy density of a commercial lithium-ion battery, which is far lower, only around 150Wh/kg.”
The battery’s high conductivity of lithium ions, and its ability to discharge and recharge 100 times, indicate that it could have great promise

































