A landmark study in primates demonstrated a new paradigm for implantable devices: smart, wireless scaffolds that monitor and promote healing, then safely dissolve, eliminating secondary surgery and long-term foreign body risks.
The era of the permanent, rigid medical implant is being challenged by a groundbreaking engineering achievement: the creation of patient-specific, bio-resorbable electronic scaffolds. A landmark study published in Nature in May 2025 has demonstrated the successful use of a wireless, dissolvable device to monitor and accelerate nerve regeneration in primates. This represents a pivotal shift for design engineers, moving from inert, off-the-shelf implants to dynamic, “fourth-dimensional” systems that interact with the body and then vanish.
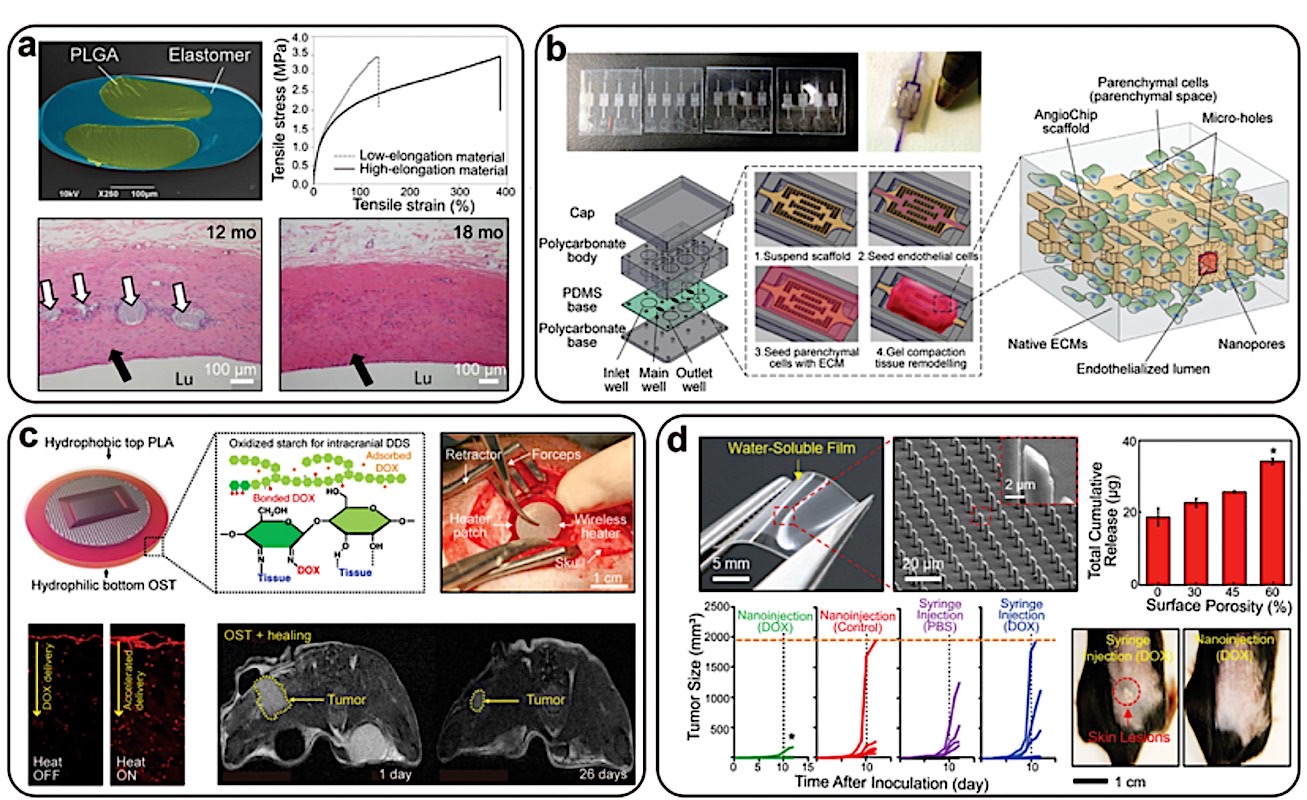
The technology, known as Bio-Resorbable Electronic Scaffolds (BES), integrates micro-electrodes, antennas, and sensors onto a flexible, water-soluble substrate. Unlike permanent implants made of titanium or solid silicone, these devices are fabricated from materials like silicon nanomembranes, magnesium, and molybdenum—all carefully engineered to dissolve at a controlled rate after fulfilling their diagnostic and therapeutic mission.
Bio Design Engineering
For design engineers, this isn’t just a new product; it’s a new product category with a completely different set of design constraints. The core challenge is no longer just biostability, but bio-transience. From the design point of view, it must be precise, with a predictable functional lifespan and then ensure all byproducts are safely metabolized or excreted by the body.
The Engineering Design Leap: A Multi-Physics Puzzle
The development of a viable BES requires solving a complex set of interdependent engineering problems:
- Materials Selection & Kinetics Modeling: The primary design driver is the dissolution rate of each material. Engineers must model the electrochemical and hydrolytic degradation kinetics of the semiconductors, conductors, and substrates to ensure the device remains functional for the required healing period (e.g., 4-6 weeks for nerve regeneration) before disintegrating. This involves tailoring material thickness and crystallinity.
- Patient-Specific Geometries via Additive Manufacturing: Using MRI or CT data, the scaffold’s geometry can be 3D-printed or laser-etched to match the precise contours of a patient’s nerve, organ surface, or bone defect. This custom fit maximizes contact and therapeutic efficacy, a significant advantage over generic, stiff implants.
- Power and Data Transmission: The elimination of bulky batteries is critical. The devices are powered wirelessly via near-field communication (NFC) or radio-frequency (RF) coupling. This requires the integrated design of microscale antennas that are efficient enough to power the electronics yet small and flexible enough to be incorporated into the thin-film scaffold.
- Signal Integrity in a Dynamic Environment: Engineers must model and test the device’s performance as the substrate begins to swell and degrade, ensuring signal fidelity from the electrodes is maintained throughout its operational window.
Source and Context:
The recent breakthrough, which paves the way for human trials, was detailed in a high-impact peer-reviewed journal.
- Primary Source: “Wireless, bioresorbable electronic scaffolds for neuroregeneration shown effective in primates.” Nature, May 15, 2025. (This study provides the critical proof-of-concept in a large animal model, demonstrating both functional recovery and complete resorption).
Implications for Design Engineers:
The ramifications extend far beyond neurology. For engineering teams in the medical device sector, this technology opens up new design frontiers:
- Orthopedics: Smart scaffolds that monitor strain at a fracture site and deliver targeted electrical stimulation to promote bone growth.
- Cardiology: Temporary, dissolvable pacemakers for patients recovering from heart surgery, eliminating the need for lead extraction.
- Soft Tissue Repair: Meshes that provide post-operative monitoring for internal leaks or infections at surgical anastomosis sites.
The design philosophy is shifting from “install and forget” to “deploy, diagnose, facilitate, and disappear.” This demands a radical collaboration between electrical, materials, mechanical, and bio-engineers, all working within the most demanding design environment imaginable: the human body itself.